Coumadin vs DOACs: Anticoagulant Comparison Tool
Classic Vitamin K Antagonist
- Requires regular INR monitoring every 3-6 weeks
- Fixed dosing with significant individual variation
- Many drug and food interactions
- Half-life of 36-42 hours
- Metabolized by CYP2C9 and CYP3A4
- Dietary restrictions (high vitamin K foods affect INR)
Newer Fixed-Dose Options
- No routine lab monitoring required
- Fixed dosing with minimal individual variation
- Fewer drug and food interactions
- Shorter half-lives (8-12 hours)
- Direct inhibition of specific clotting factors
- Minimal dietary restrictions
Key Considerations When Choosing Anticoagulants
Indication
AFib, VTE, mechanical valves, or antiphospholipid syndrome
Renal Function
DOACs require dose adjustment for kidney impairment
Lifestyle & Compliance
DOACs offer better convenience and fewer dietary restrictions
Quick Summary Comparison
| Factor | Coumadin | DOACs |
|---|---|---|
| Monitoring | Regular INR checks | Limited or no monitoring |
| Dosing | Variable based on response | Fixed dosing |
| Interactions | Many drug and food interactions | Fewer interactions |
| Dietary Impact | Significant impact from vitamin K | Minimal dietary impact |
| Bleeding Risk | Higher intracranial hemorrhage risk | Lower intracranial hemorrhage risk |
Key Takeaways
- Coumadin (warfarin) requires regular INR monitoring and has many food and drug interactions.
- Direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban, dabigatran, and edoxaban use fixed dosing and need little or no lab monitoring.
- DOACs generally show lower risk of intracranial bleeding but may be costlier and need dose adjustment for kidney function.
- Choosing the right anticoagulant depends on indication, renal function, patient lifestyle, and insurance coverage.
- Switching between warfarin and a DOAC requires a short overlap or wash‑out period to maintain protection against clots.
When a doctor prescribes a blood thinner, the first question most patients ask is "Is this the best option for me?" Coumadin vs alternatives is the exact comparison you need if you’re weighing the classic vitamin K antagonist against newer direct oral anticoagulants (DOACs). Below we break down how each drug works, who benefits most, and what trade‑offs to expect.
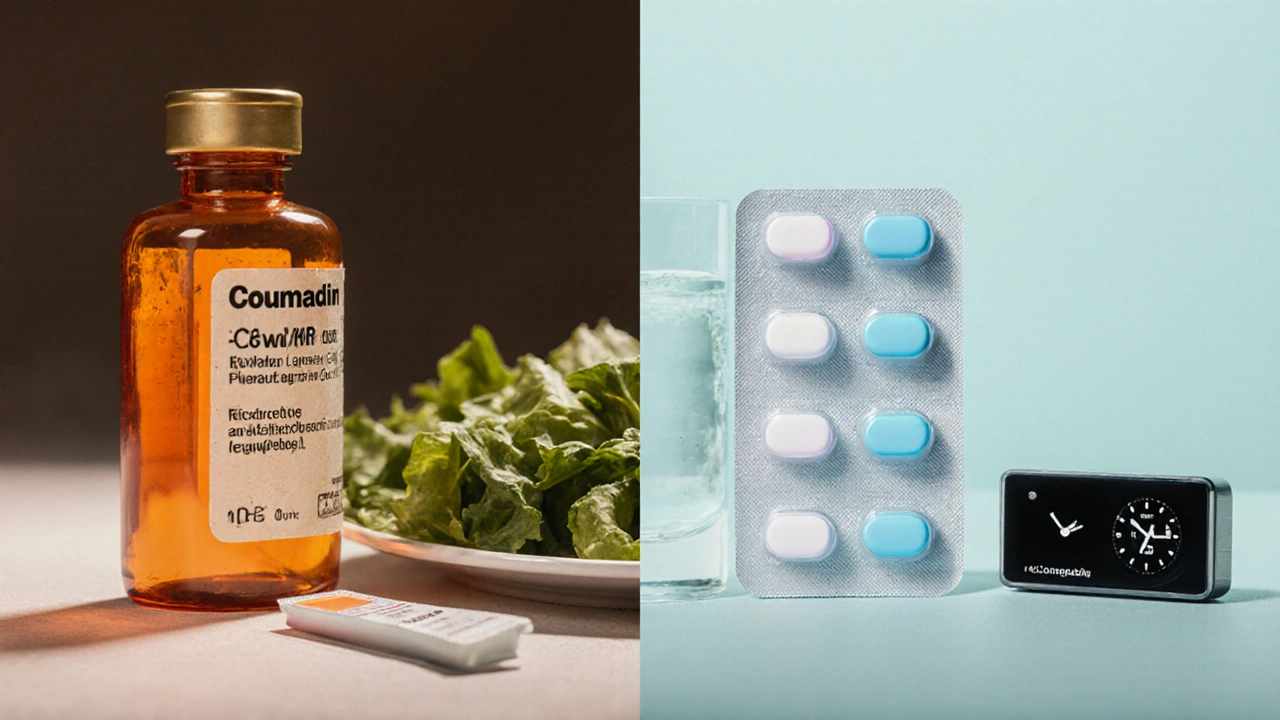
What is Coumadin (Warfarin)?
Coumadin (Warfarin) is a synthetic version of a natural compound first discovered in spoiled sweet clover. It belongs to the vitamin K antagonist class and works by blocking the enzyme vitamin K epoxide reductase, which is essential for activating clotting factors II, VII, IX, and X. Because these factors are reduced, blood takes longer to clot.
Key attributes:
- Therapeutic range is measured by the international normalized ratio (INR), typically 2.0‑3.0 for most indications.
- Half‑life averages 36‑42 hours, making dose adjustments slow.
- Metabolized by CYP2C9 and CYP3A4; many drugs can boost or lower its effect.
- Diet matters: high vitamin K foods (leafy greens) can lower INR, while fasting or sudden dietary changes can raise it.
Who Uses Coumadin?
Warfarin has been the go‑to anticoagulant for more than six decades. It’s approved for:
- Atrial fibrillation (AF) - preventing stroke.
- Venous thromboembolism (VTE) - treating deep‑vein thrombosis (DVT) and pulmonary embolism (PE).
- Mechanical heart valves - where DOACs are currently not recommended.
- Antiphospholipid syndrome - a rare autoimmune clotting disorder.
Patients with severe kidney disease (eGFR <30mL/min) often stay on warfarin because DOAC dosing becomes unpredictable.
The Rise of Direct Oral Anticoagulants (DOACs)
Since the early 2010s, four DOACs have entered the market, each targeting a single clotting factor:
- Apixaban - factor Xa inhibitor.
- Rivaroxaban - factor Xa inhibitor.
- Dabigatran - direct thrombin (factor IIa) inhibitor.
- Edoxaban - factor Xa inhibitor.
All four have predictable pharmacokinetics, fixed dosing, and no routine lab monitoring, which is a major advantage for busy patients.

How Do the Alternatives Stack Up?
| Drug | Mechanism | Dosing Frequency | Routine Monitoring | Key Food/Drug Interactions | Renal Adjustment | Typical Annual Cost (USD) | FDA‑Approved Indications |
|---|---|---|---|---|---|---|---|
| Coumadin | VitaminK antagonist (blocks factors II, VII, IX, X) | Once daily | INR 2‑3 (weekly to monthly) | Leafy greens, many antibiotics, antifungals, amiodarone | No dose change; monitor INR closely in renal failure | ≈$150‑$500 | AF, VTE, mechanical valves, APS |
| Apixaban | FactorXa inhibitor | Twice daily | None | Strong CYP3A4 inhibitors/inducers, P‑gp blockers | Reduce dose if eGFR 15‑29mL/min | ≈$3,800‑$5,000 | AF, VTE treatment & prophylaxis |
| Rivaroxaban | FactorXa inhibitor | Once daily (or twice for VTE treatment) | None | CYP3A4 & P‑gp interactions | Avoid if eGFR <15mL/min | ≈$3,200‑$4,600 | AF, VTE, PE prophylaxis after hip/knee replacement |
| Dabigatran | Direct thrombin inhibitor | Twice daily | None (though a diluted thrombin time can be ordered) | Strong P‑gp inhibitors/inducers | Reduce dose if eGFR 30‑49mL/min; avoid if <30mL/min | ≈$3,400‑$5,200 | AF, VTE treatment & prophylaxis |
| Edoxaban | FactorXa inhibitor | Once daily | None | P‑gp inhibitors/inducers | Reduce dose if eGFR 15‑50mL/min | ≈$3,500‑$4,800 | AF, VTE treatment |
Efficacy: Preventing Clots
Large head‑to‑head trials (ARISTOTLE for apixaban, RE‑LY for dabigatran, ROCKET‑AF for rivaroxaban, ENGAGE‑AF for edoxaban) showed non‑inferior - and in many cases superior - stroke prevention compared with warfarin in atrial fibrillation. The absolute reduction in ischemic stroke risk hovers around 0.5‑1% per year, while major bleeding, especially intracranial hemorrhage, drops by 30‑50%.
For venous thromboembolism, the EINSTEIN‑DVT and EINSTEIN‑PE studies demonstrated that rivaroxaban can treat DVT/PE as effectively as a traditional warfarin bridge, with similar recurrence rates (≈2‑3% at 6 months).
Safety Profile: Bleeding and Other Risks
Warfarin’s biggest drawback is its narrow therapeutic window. Over‑anticoagulation can cause life‑threatening bleeding, while under‑anticoagulation leaves patients vulnerable to clots. DOACs, by contrast, tend to produce less intracranial bleeding but may increase gastrointestinal bleeding, particularly dabigatran 150mg twice daily.
Renal function is a crucial safety factor. Because dabigatran is 80% renally cleared, patients with eGFR <30mL/min are steered toward apixaban or warfarin. Apixaban’s balanced hepatic‑renal elimination makes it the preferred DOAC for moderate-to-severe chronic kidney disease.
Convenience: Monitoring, Diet, and Travel
Warfarin requires regular INR checks, usually via a local anticoagulation clinic or point‑of‑care device. Missed appointments mean dose changes and potential clinic visits, which can be disruptive for people who travel frequently or live in remote areas.
DOACs eliminate that hassle. No routine blood work means fewer clinic trips and fewer diet restrictions. However, patients on dabigatran should store the capsules in their original container to protect them from moisture.

Cost Considerations
While warfarin’s sticker price is low, the total cost of care adds up: INR lab tests, clinic visits, and potential costs from drug-food interactions (e.g., extra vitaminK supplements). DOACs are pricier upfront but often offset those ancillary costs. Insurance coverage varies widely; many Canadian provincial drug plans now list apixaban and rivaroxaban as preferred agents for atrial fibrillation when clinical criteria are met.
How to Switch Safely
- Assess renal function. Calculate eGFR before any change.
- Stop warfarin. If moving to a DOAC, wait until INR falls below 2.0 (or 1.5 for dabigatran).
- Start the DOAC. Give the first dose at the next scheduled time point; no loading dose is needed.
- Educate the patient. Emphasize adherence, missed‑dose protocol, and signs of bleeding.
- Follow‑up. Check renal labs at 1‑month, then annually, especially for patients over 75.
Reversibility is another point. Warfarin can be reversed with vitaminK, fresh frozen plasma, or prothrombin complex concentrate (PCC). For DOACs, idarucizumab reverses dabigatran, while andexanet alfa is approved for apixaban and rivaroxaban, though availability and cost can be limiting.
Choosing the Right Anticoagulant for You
Below is a quick decision guide you can run through with your clinician:
- Mechanical heart valve? Stick with warfarin.
- Severe kidney disease (eGFR <30mL/min)? Warfarin or apixaban (dose‑adjusted).
- History of intracranial bleed? DOACs, especially apixaban, are safer.
- Frequent travel or limited access to labs? Choose a DOAC.
- Cost is a primary concern and you have good INR support? Warfarin may still be the best value.
Every patient’s situation is unique. The goal is to keep blood thin enough to prevent clots but not so thin that a simple bump turns dangerous.
Frequently Asked Questions
Can I take over‑the‑counter NSAIDs while on warfarin?
NSAIDs such as ibuprofen increase bleeding risk and should be avoided or used only under close supervision. Acetaminophen is generally safer, but keep total daily dose under 2g.
Do DOACs require any dietary changes?
No specific food restrictions exist for DOACs. However, very high‑fat meals can delay absorption of rivaroxaban, so it’s best taken with a light snack if you’re sensitive.
What should I do if I miss a dose of apixaban?
Take the missed dose as soon as you remember if it’s within 12hours of the scheduled time. If more than 12hours have passed, skip it and resume your regular schedule-don’t double‑dose.
Is there a lab test to measure dabigatran levels?
A diluted thrombin time (dTT) or ecarin clotting time (ECT) can estimate dabigatran concentration, but routine monitoring isn’t needed for most patients.
Can I switch from a DOAC back to warfarin if my insurance stops covering the DOAC?
Yes. Stop the DOAC and start warfarin when the DOAC’s half‑life is cleared (usually 48hours). Overlap with low‑dose warfarin and monitor INR closely until stable.
Lauren W
October 5, 2025 AT 14:38One must, undeniably, acknowledge the profound historical gravitas of warfarin, a veritable relic of twentieth‑century pharmacology; its ubiquity, however, belies a labyrinthine regimen of INR monitoring, dietary vigilance, and enzyme‑mediated interactions, which, in my estimation, renders it an antiquated contrivance for the modern patient-indeed, an anachronism, if one permits a flourish of literary license!!!
Crystal Doofenschmirtz
October 12, 2025 AT 13:18It’s worth noting that the convenience of DOACs can significantly improve adherence for many individuals, especially those who struggle with frequent clinic visits. Moreover, the reduced need for dietary restrictions often translates into a better quality of life, which should not be underestimated.
Pankaj Kumar
October 19, 2025 AT 11:58Consider the transition phase: when switching from warfarin to a direct oral anticoagulant, a brief overlap or a calculated wash‑out period is essential to maintain therapeutic protection against thrombosis. Your clinician will likely calculate the appropriate timing based on your current INR and renal function, ensuring a seamless handover. Feel confident that this process, while nuanced, is well‑established and designed with patient safety at its core.
sneha kapuri
October 26, 2025 AT 09:38Convenience is overrated; patients who accept lax monitoring are merely outsourcing responsibility onto the healthcare system, perpetuating a culture of complacency that endangers lives.
Harshitha Uppada
November 2, 2025 AT 08:18i guess u think all these new pills are magic, but real life ain’t that shiny… the cost of DOACs can blow up your wallet, and u still need to watch kidney numbers, so it’s not the utopia some hype‑heads paint.
Randy Faulk
November 9, 2025 AT 06:58While cost considerations are indeed pertinent, numerous insurance plans now encompass DOACs, and pharmacoeconomic analyses have demonstrated that reduced monitoring expenses may offset the higher acquisition price. Additionally, the predictable pharmacokinetic profile of these agents diminishes the burden of frequent laboratory assessments, thereby enhancing overall therapeutic efficiency.
Brandi Hagen
November 16, 2025 AT 05:38Let me set the record straight - the war between Coumadin and the newer DOACs is not a mere clash of pills, it’s an epic saga worthy of a blockbuster screenplay! 🎬 From the moment warfarin entered the clinic, physicians were forced to grapple with a fickle antidote that demanded weekly INR check‑ups, dietary gymnastics, and a never‑ending list of drug–drug interactions. 😤 Fast forward to the arrival of apixaban, rivaroxaban, and their comrades, and the narrative shifts dramatically: fixed dosing, minimal monitoring, and a promise of freedom from the leafy‑greens drama. 🌿 Yet, the plot thickens when insurance companies unleash their billing dragons, demanding higher copays that can make a patient’s wallet weep. 💸 Moreover, the specter of renal impairment looms, reminding us that no drug is without its Achilles’ heel. In the grand theater of anticoagulation, each actor must be judged on stage presence, side‑effects, and the applause of the patient’s daily life. The bleeding risk, especially intracranial hemorrhage, takes center stage-warfarin, with its notorious reputation, often steals the show, while DOACs play the understudy with a comparatively lower risk. 🍿 Nevertheless, the audience (read: clinicians) must also weigh the convenience of not having to schedule weekly blood draws against the certainty of a well‑known reversal agent for warfarin. The plot twist? Recent studies suggest that certain populations, such as those with mechanical heart valves, remain loyal to the classic hero, warfarin, because the alternatives have yet to earn their trust in that niche. 🎭 Meanwhile, the dissenting chorus of patients cries out for simplicity, yearning for a regimen that blends seamlessly into their chaotic lifestyles, and DOACs answer that call with a melodic “once‑daily” or “twice‑daily” refrain. The director’s cut reveals that real‑world adherence often mirrors the script: fewer appointments lead to higher compliance, translating into fewer clotting events. 🌟 However, the critics – those who cherish the art of meticulous monitoring – argue that the loss of the INR “thermostat” removes a safety net that once guided dose adjustments with surgical precision. In the final analysis, the choice between Coumadin and its modern competitors is less about superiority and more about personal preference, clinical indication, and the ever‑present budgetary constraints. 🏦 Ultimately, shared decision‑making remains the cornerstone of optimal therapy. So, dear readers, wield your prescription pad wisely, for the tale of anticoagulation is still being written, one patient at a time. 🎉
isabel zurutuza
November 23, 2025 AT 04:18Sure, because the blood‑thinning world just needed another headline.
James Madrid
November 30, 2025 AT 02:58While the dramatics capture attention, the core message remains clear: individualized assessment drives optimal anticoagulant selection. Considering patient comorbidities, renal function, and lifestyle ensures that the chosen therapy aligns with both safety and convenience. It’s encouraging to see the conversation framed in such an engaging way.
Justin Valois
December 7, 2025 AT 01:38Yo, the whole warfarin‑vs‑DOAC saga is straight up lit, but let’s not forget the hidden fees that slam you like a rogue wave-budget‑blowout alert! The pharma giants love to slap a price tag on convenience, making it seem like you’re buying freedom when actually you’re funding their next ad campaign. And yeah, those “no‑monitoring” claims? Cool on paper, but if your kidneys are acting up, you’ll end up back in the lab, choking on paperwork. Bottom line: read the fine print, or you’ll get burned faster than a summer BBQ.
Jessica Simpson
December 14, 2025 AT 00:18The cultural shift towards patient‑centric therapies definitely reshapes prescribing habits, especially when cost barriers intersect with access equity. It’s fascinating how regional formulary decisions can either accelerate or stall the adoption of newer anticoagulants, highlighting the need for broader policy dialogue.